Cysteine proteases in plant immunity
We identified an apoplastic maize cystatin (CC9; corn cystatin9) to be highly induced at the early biotrophic interaction with U. maydis wild type but not upon infection of the incompatible pep1 deletion mutant. VIGS of CC9 revealed that it is essential for U. maydis infection. In cystatin silenced plants, U. maydis infection is terminated by plant cell death (van der Linde et al., 2012, Plant Cell). As trigger for this plant defense response we identified a set of apoplastic cysteine proteases (ACPs) that can be activated by salicylic acid (SA) treatment and all these proteases can be inhibited by the cystatin. Consequently, the cystatin was found to block SA-triggered defense including cell death when being present in the apoplast. We now aim to elucidate which roles the individual ACPs have in maize defense. To identify the signals that trigger SA-associated plant defense responses we are particularly interested in the targets of these proteases.
Another perspective on the role of apoplastic cysteine proteases comes from our studies on the Pit2 effector, which targets the same maize enzymes that were identified in course of the CC9 project. Inhibition of ACPs by Pit2 results in suppression of defense responses and enhanced disease symptoms. Pit2 has a protease inhibitor domain (PID14) able to inhibit ACPs. PID14 is conserved in different smut fungi. The characterization of PID14 as a common motif for ACPs inhibition as well as its mechanism of action has a main focus on our research.
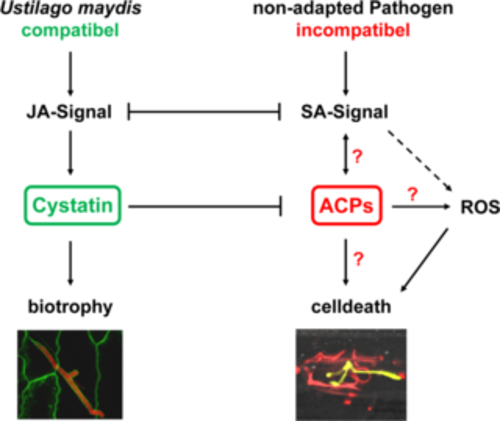
Figure 4: Induction of maize defense by apoplastic cysteine proteases (ACPs) and their inhibition by a maize cystatins (CC). SA-associated defense induction is activated via ACPs and finally leads to plant cell death. In a biotrophic interaction, JA signaling activates cystatin expression. This inhibits activation of ACPs and thereby blocks plant defense. JA: Jasmonic Acid; SA: Salicylic Acid; ROS: Reactive Oxygen Species;?: ACP targets are unknown.